CDISC Migration:
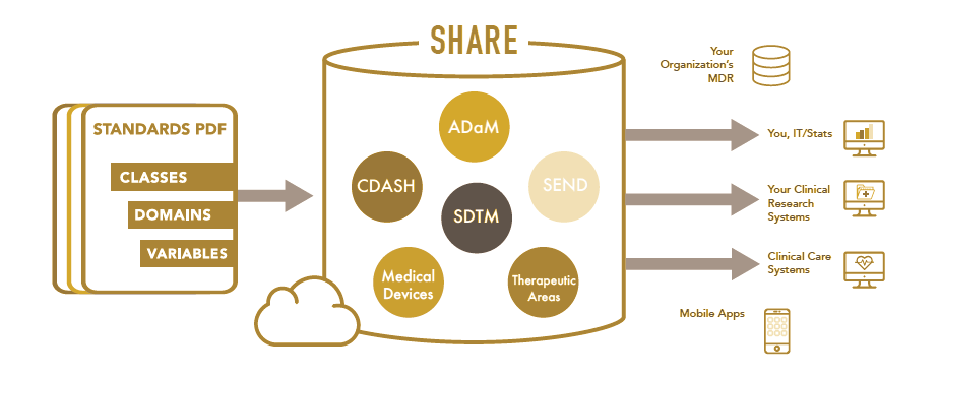
Understanding and complying with CDISC data standards is critical to satisfying regulatory data submission requirements. Our experienced programmers and consultant team have a strong technical understanding in Study Data Tabulation Model (SDTM) analysis Data Model (ADaM), CDISC terminologies, corresponding implementation and reviewers guide and the related applicable regulations.
We design Clinical Data Acquisition Standards Harmonization (CDASH) compliant CRFs for paper based and electronic data capture (EDC) clinical trials. The integration of CDASH standard compatible CRFs allows us to propose our clients with SDTM compliant data models in the early phase of development.
Our team utilizes active research coupled with technicalities to understand the requirement and comply with adherence to the Clinical Data Interchange Standards Consortium (CDISC) data standards.
Our professional teams integrate their deep understanding of the guidelines for the standardization, improved data statistical programming capabalities like Analysis Data Model (ADM) and integrated tools for delivering the deliverables efficently.
CDISC Package Creation Services:
- Preparing Annotated CRF as per standard guidelines.
- SDTM dataset creation.
- ADaM datasets creation.
- SDRG Creation .
- ADRG Creation .
- Define.XML Creation.
- Data validation of SDTM & AdaM.
CDISC Package Review Services:
- Annotated CRF Review.
- SDTM datasets review.
- ADaM datasets review of SDRG review and ADRG review.
- Define.XML review.